TRIUMPH OLE
TYVASO demonstrated long-term treatment benefit2
TRIUMPH OLE Study Design: An open-label extension (OLE) of the TRIUMPH study to evaluate the safety and dosing of TYVASO over time. Patients (N=206) from the placebo-controlled 12-week TRIUMPH study initiated TYVASO and entered the long-term, uncontrolled OLE study. NYHA FC II (11%), III (86%), IV (3%) at baseline.2,3
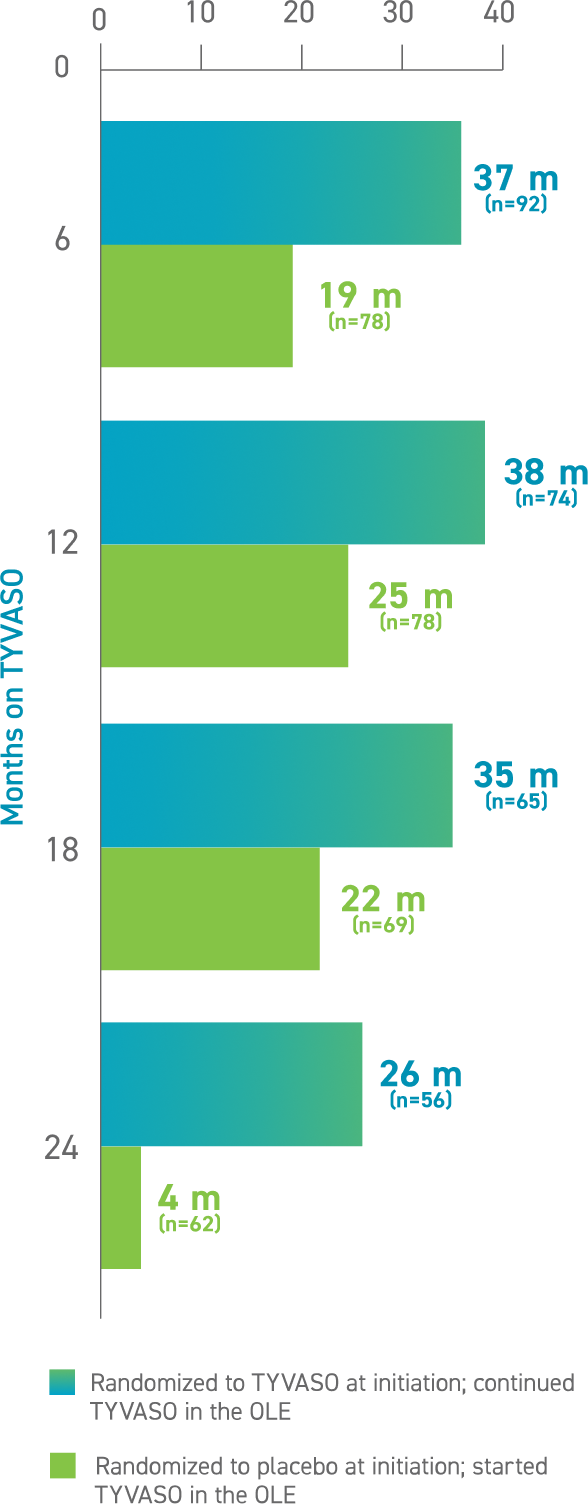
Median changes in 6MWD at 6, 12, 18, and 24 months were 28, 31, 32, and 18 meters, respectively, for all participants, inclusive of the significant attrition in median change in walk distance for those patients who initially received the placebo.2
Without a control group, data must be interpreted cautiously.
2x
The percentage of patients who walked greater than 440 meters, a reported positive predictor of survival, increased from 13% (N=15) at baseline to 26% (N=31) at 24 months.2
Quality of life and health biomarkers in TRIUMPH OLE
Borg dyspnea scores improved throughout the 2-year study, and by a significant margin at month 6 (–0.37; P<0.02)2
Patients completing the MLWHF questionnaire reported significant improvements in all dimensions (physical, global, and emotional) through 24 months2
At 24 months, 69% of patients had not experienced a clinical worsening event2*
*Clinical worsening was defined as addition of a new PAH therapy or discontinuation due to disease progression or death.2
H-L=Hodges-Lehmann; MLWHF=Minnesota Living with Heart Failure.
Long-term benefits beyond 6MWD
SUSTAINED IMPROVEMENTS IN NYHA FUNCTIONAL CLASS2

maintained or improved NYHA FC from baseline at 2 years (n=120)1,2
36% of patients had improvement in functional class from baseline at 6 months (n=174) and at 2 years (n=120).1,2
SURVIVAL EXCEEDED 80% AT 3 YEARS3

survival rate at 3 years (n=69)3
Based on a long-term follow-up of patients who were treated with TYVASO in the pivotal study and the OLE (Kaplan–Meier estimates of survival).3
Data must be interpreted cautiously and cannot be used to determine the long-term effect on functional class or mortality.
Safety from the OLE study
- AEs observed during this chronic dosing study were qualitatively similar to those observed in the 12-week placebo-controlled trial3

