TYVASO Hemodynamics Study—assessing hemodynamics, 6MWD, safety, and pharmacokinetics2*
Study design
In a multicenter, nonrandomized, open-label, single-arm study, the efficacy of TYVASO* on hemodynamics and exercise capacity, safety, and pharmacokinetics was evaluated in 17 Japanese patients with PAH.2
The initial study lasted 12 weeks, and a 40-week, long-term treatment period was available for patients who wanted to continue taking TYVASO. Of the 17 patients who participated in the initial study, 16 entered the long-term extension and continued taking TYVASO until week 52.2
- Patients initiated TYVASO at 3 breaths per session, 4 times daily, with a maximum of 9 breaths per session, 4 times daily2
- If tolerated, the dosage was gradually increased with a minimal interval of 7 days2
- Delivered via the TYVASO nebulizer2
Patient eligibility
-
Adult Japanese patients with PAH2
- Hemodynamic criteria for PAH: mPAP ≥25 mm Hg, PAWP ≤15 mm Hg, PVR ≥5 WU2
- Patients had either IPAH, HPAH, drug- or toxin-induced PAH, or PAH associated with CTD or HIV2
- Baseline 6MWD ≥200 m2
- If on an ERA and/or PDE-5 inhibitor or sGC stimulator, must have initiated >12 weeks prior to the trial and been on a stable dose for >4 weeks2
- Could not currently be taking a prostacyclin2
*Additional parameters measured include WHO FC, NT-proBNP, Borg Dyspnea Score, and an MLWHF QOL questionnaire.2
Evaluating the impact of TYVASO on hemodynamic parameters2
Study endpoints
Primary Endpoint2
- Change in PVRI from baseline to week 12*
Secondary Endpoints2
- Other hemodynamic parameters, including PVR, mPAP, CO, and CI
- Peak 6MWD
- WHO FC
- NT-proBNP levels
- Time to clinical worsening, defined as death, transplantation, hospitalization due to worsening PAH, or initiation of additional PAH-specific therapy
- Borg Dyspnea Score
- MLWHF QOL
Safety Endpoints2
- Adverse events
- Pulmonary function test
- Vital signs (blood pressure, pulse, and SpO2)
- Electrocardiogram
- Chest X‐ray
- Arterial blood gas analysis
- Body weight
- Routine laboratory parameters measured in a clinical laboratory
Pharmacokinetics2
-
PK parameters, including†:
- Cmax: maximum plasma concentration
- AUClast: AUC from time 0 to last measurable concentration sampling time
- T1/2: elimination half-life
*The lower PVRI measured at 15 or 30 minutes after inhalation was taken as the designated “best” PVRI at week 12.2
†Determined from the plasma concentration-time curve.2
AUC=area under the curve; CI=cardiac index; CO=cardiac output; PK=pharmacokinetics; PVRI=pulmonary vascular resistance index; SpO2=percutaneous arterial oxygen saturation.
Patients were primarily FC II with a mean 6MWD above the low-risk threshold
Study patient characteristics (N=17)2
88.2%
had IPAH or HPAH
65%
were FC II
487.8
± 112.4 m
mean 6MWD
| Patient demographic data1,2 | N=17 |
|---|---|
| Female, n (%) | 10 (58.8) |
| Age, mean ± SD, y | 46.4 ± 15.5 |
| Time since PAH diagnosis, mean ± SD, y | 4.0 ± 4.6 |
| NT-proBNP, mean ± SD, pg/mL | 144.1 ± 213.3 |
| PAH classification, n (%) | |
| IPAH/HPAH | 15 (88.2) |
| Associated with connective tissue disease | 2 (11.8) |
| Baseline WHO FC, n (%) | |
| II | 11 (64.7) |
| III | 6 (35.3) |
| Background PAH therapy, n (%) | |
| No | 1 (5.9) |
| Yes | 16 (94.1) |
| ERA | 3 (17.6) |
| ERA and PDE-5 inhibitor/sGC stimulator | 13 (76.5) |
| Previous prostacyclin therapy, n (%) | |
| No | 8 (47.1) |
| Yes | 9 (52.9) |
| Selexipag (oral) | 7 (41.2) |
| Bereprost (oral) | 2 (11.8) |
| Iloprost (inhalation) | 1 (5.9) |
SD=standard deviation.
Changes to hemodynamics and other measures after 12 weeks with TYVASO2
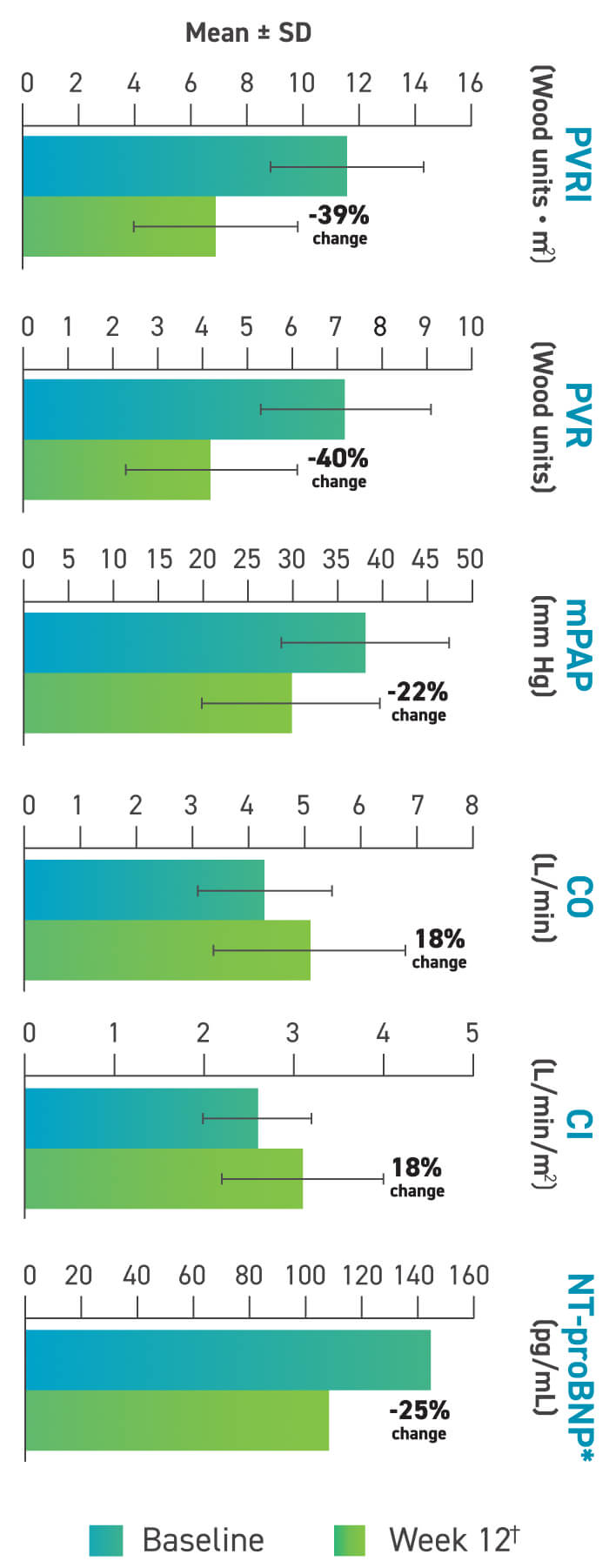
Other hemodynamic changes:
- SVR and SVRI numerically improved2
There were no significant changes in PAWP, mRAP, or SvO2.2
These data are from a single-arm, uncontrolled study. Without a control group, data must be interpreted cautiously.
How could the assessment of key early indicators of PAH impact later indicators for your patients?
*Data points for NT-proBNP graph, including standard error: baseline, 144.1±213.3 pg/mL; week 12, 108.6±165.6 mg/mL.2
†The lower PVRI measured at 15 or 30 minutes after inhalation was taken as the designated “best” PVRI at week 12.2
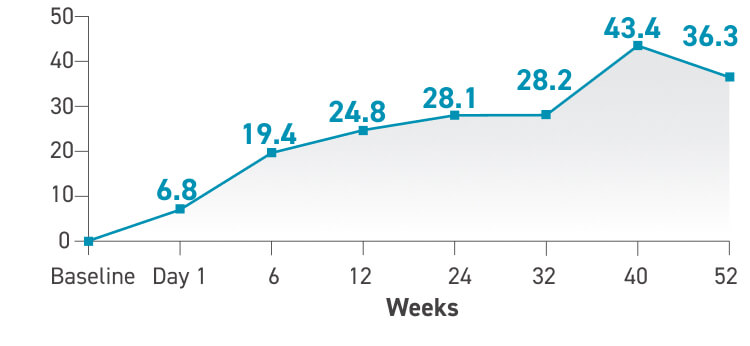
Changes in 6MWD and FC through 1 year2*†‡
Changes in 6MWD seen as early as day 1 and sustained at 1 year2
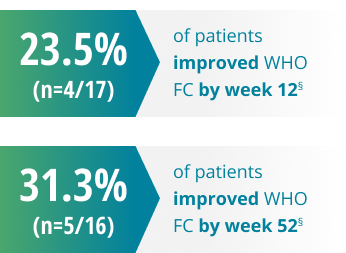
Changes in WHO FC2

These data are from a single-arm, uncontrolled study. Without a control group, data must be interpreted cautiously.
*Of the 17 patients who participated in the initial 12-week study, 16 continued taking TYVASO for an additional 40 weeks.2
†No significant changes in Borg Dyspnea Score at weeks 6, 12, or 52.2
‡QOL had mean changes from baseline in the global score of −6.6 and −5.8 at weeks 12 and 52, respectively.2
§13 out of 17 patients maintained their baseline FC at week 12, and 11 out of 16 patients maintained their FC by week 52.2
SAFETY PROFILE SIMILAR TO ESTABLISHED TYVASO AEs2
Summary of AEs over 52 weeks2
| AEs related to treprostinil (reported ≥ 10%) | |
|---|---|
| Headache | 10 (58.8) |
| Cough | 8 (47.1) |
| Throat irritation | 5 (29.4) |
| Hot flush | 4 (23.5) |
| Nausea | 3 (17.6) |
| Head discomfort | 3 (17.6) |
| Oropharyngeal discomfort | 3 (17.6) |
| Dizziness | 2 (11.8) |
| Oropharyngeal pain | 2 (11.8) |
| Blood pressure decreased | 2 (11.8) |
| Pyrexia | 2 (11.8) |
| Diarrhea | 2 (11.8) |
| Palpitations | 2 (11.8) |
No patients died, had a transplant, or were hospitalized due to worsening PAH at any point during the study.
No patient needed additional PAH-specific therapy in the main treatment period.
- 2 patients on an ERA received add‐on therapy with a PDE-5 inhibitor or sGC stimulator in the long‐term treatment period2
These data are from a single-arm, uncontrolled study. Without a control group, data must be interpreted cautiously.

