FREQUENTLY ASKED QUESTIONS
GENERAL TYVASO QUESTIONS
What is TYVASO?
How was TYVASO studied for the treatment of PH-ILD?
What are the most common adverse events?
Can TYVASO be used during pregnancy?
Are there any data on long-term treatment with TYVASO for PH-ILD?
TYVASO NEBULIZER QUESTIONS
What is the TYVASO nebulizer?
How long does each dosing session take with the TYVASO nebulizer?
How do patients keep track of their treatments?
How is the TYVASO nebulizer cleaned?
How do you order and replace the accessories?
How is TYVASO titrated?
TYVASO DPI QUESTIONS
What is TYVASO DPI?
Was TYVASO DPI studied in patients with PH-ILD?
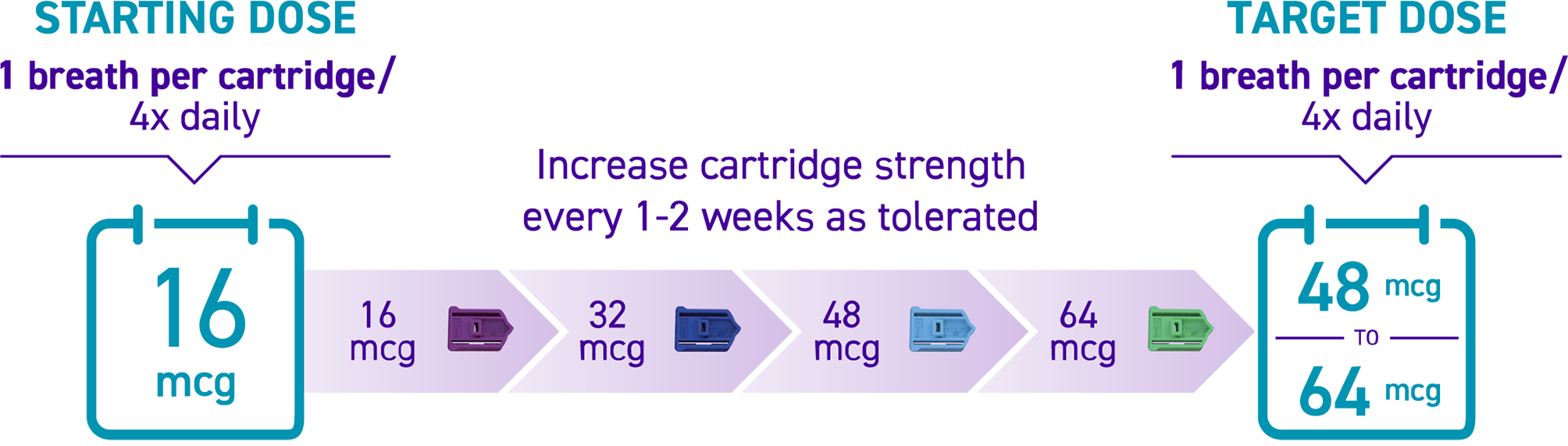
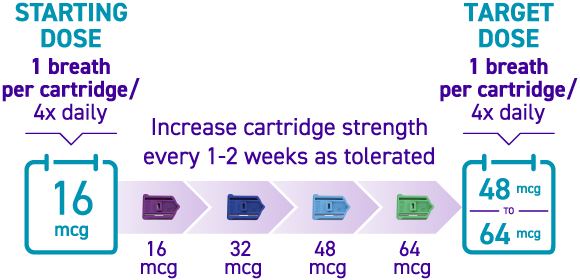
How is TYVASO DPI titrated?
TYVASO SUPPLY AND STORAGE QUESTIONS
How is TYVASO supplied?
How is TYVASO stored?
How is TYVASO distributed?
What additional support can United Therapeutics provide my patients?
What additional support does the Specialty Pharmacy provider offer your patients?
What is a UT Regional Nurse Specialist (RNS) and how do I contact them?
AE=adverse event; DPI=dry powder inhaler; PAH=pulmonary arterial hypertension; WHO=World Health Organization.
The information contained in this section of the site is clinical in nature and specifically created for healthcare professionals. If you are not a US healthcare professional, please click CLOSE to return to the consumer section of the site.