BREEZE STUDY
The BREEZE study was designed to assess the safety and tolerability of switching from TYVASO to TYVASO DPI1
BREEZE Study Design: This open-label clinical study evaluated patients with PAH (N=51) who were on a stable dose of TYVASO and were switched to a corresponding dose of TYVASO DPI.1
Patients underwent safety and pharmacokinetic assessments and
49 of 51 patients (96%) elected to continue therapy with TYVASO DPI in an optional extension phase (OEP) with follow-up visits every 8 weeks.1
Select patient background information
- Patients started TYVASO ≥3 months prior to the baseline visit and were on a stable regimen (no change in dose within 30 days of baseline visit) of TYVASO (6 to 12 breaths QID)1
-
80% of patients were on dual background therapy in addition to TYVASO1*
- If receiving other approved background therapy, the patient was required to be on a stable dose with no additions or discontinuations for a minimum of 30 days1
- 61% of patients were FC II at screening1†
- FC IV patients were excluded from the study2
- Patients had a mean baseline 6MWD of 418.9 m (SD 109.4)1
- Patients had a forced expiratory volume in 1 second (FEV1) ≥60% and forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) ratio ≥60% during the 6 months prior to enrollment1
Results
Adverse reactions: Consistent safety and tolerability when switching to TYVASO DPI3
During the treatment phase of the BREEZE study, most commonly reported (≥4% of patients) AEs in patients previously stable on TYVASO were1,3:
Discontinuation of TYVASO DPI due to an AE occurred in 2 (3.9%) patients.1
Patient tolerability, as assessed by incidence of new adverse events following transition to TYVASO DPI, was consistent with the expected known safety profile of TYVASO.3
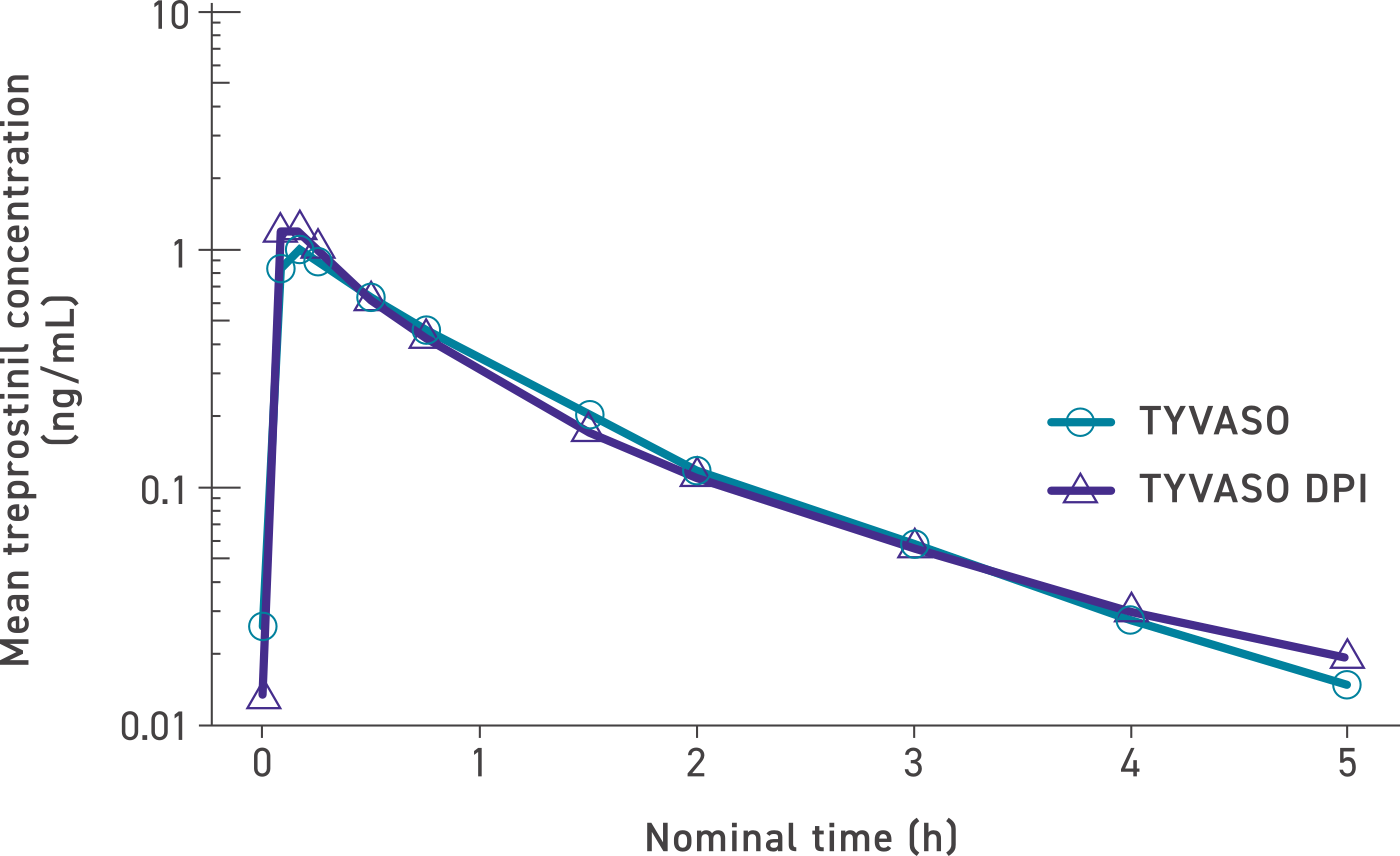
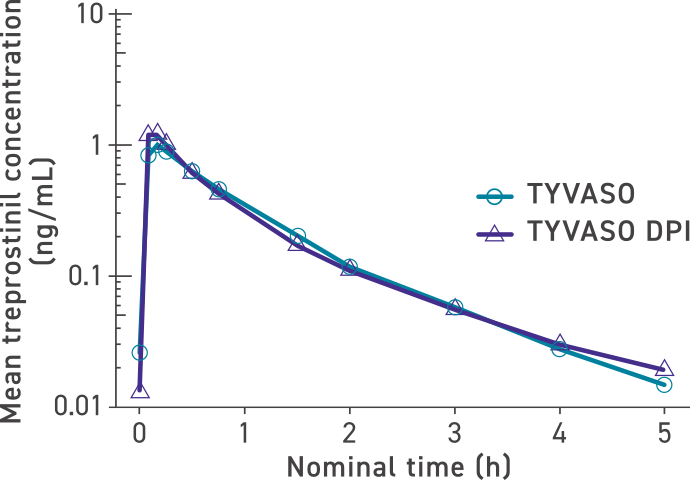
Systemic exposure was similar between TYVASO DPI and TYVASO1,4
Mean treprostinil concentration over time (dose levels pooled)4
The maximum mean treprostinil concentration occurred rapidly for both TYVASO and TYVASO DPI in the low-, mid-, and high-dose groups: 32 mcg (

11.5 m additional increase in 6MWD1
For patients previously stable on TYVASO, TYVASO DPI demonstrated a significant improvement in 6MWD at week 3 compared with TYVASO treatment at baseline. Interim results of the OEP suggest that this improvement was sustained over 51 weeks.1
Satisfaction with TYVASO DPI significantly improved when compared with TYVASO1

45/46 (P<0.0001)
REPORTED PATIENT SATISFACTION WITH TYVASO DPI
at week 3 compared with 31% (16/51) of patients satisfied with the TYVASO ultrasonic nebulizer at baseline.1
TYVASO DPI is a simple-to-use delivery option
6MWD=6-minute walk distance; AE=adverse event; DPI=dry powder inhaler; FC=functional class; PAH=pulmonary arterial hypertension; QID=4 times daily; SD=standard deviation.
The information contained in this section of the site is clinical in nature and specifically created for healthcare professionals. If you are not a US healthcare professional, please click CLOSE to return to the consumer section of the site.