DOSING & TITRATION
WHY TYVASO?
SO
titration to higher doses is simple2
TYVASO DPI dosing & titration
TYVASO DPI is dosed with just 1 breath, 4x daily, approximately every 4 waking hours. Each dose is inhaled in less than 2 seconds.2,3
Titration with TYVASO DPI2

Titration schedules may vary based on tolerability. The target maintenance dose is usually 48 to 64 mcg per session. If the prescribed dose is higher than 80 mcg per treatment session, more than 1 cartridge will be needed per session.2
Dose comparison2,4
Patients currently using the TYVASO nebulizer can be switched to the corresponding dose of TYVASO DPI:
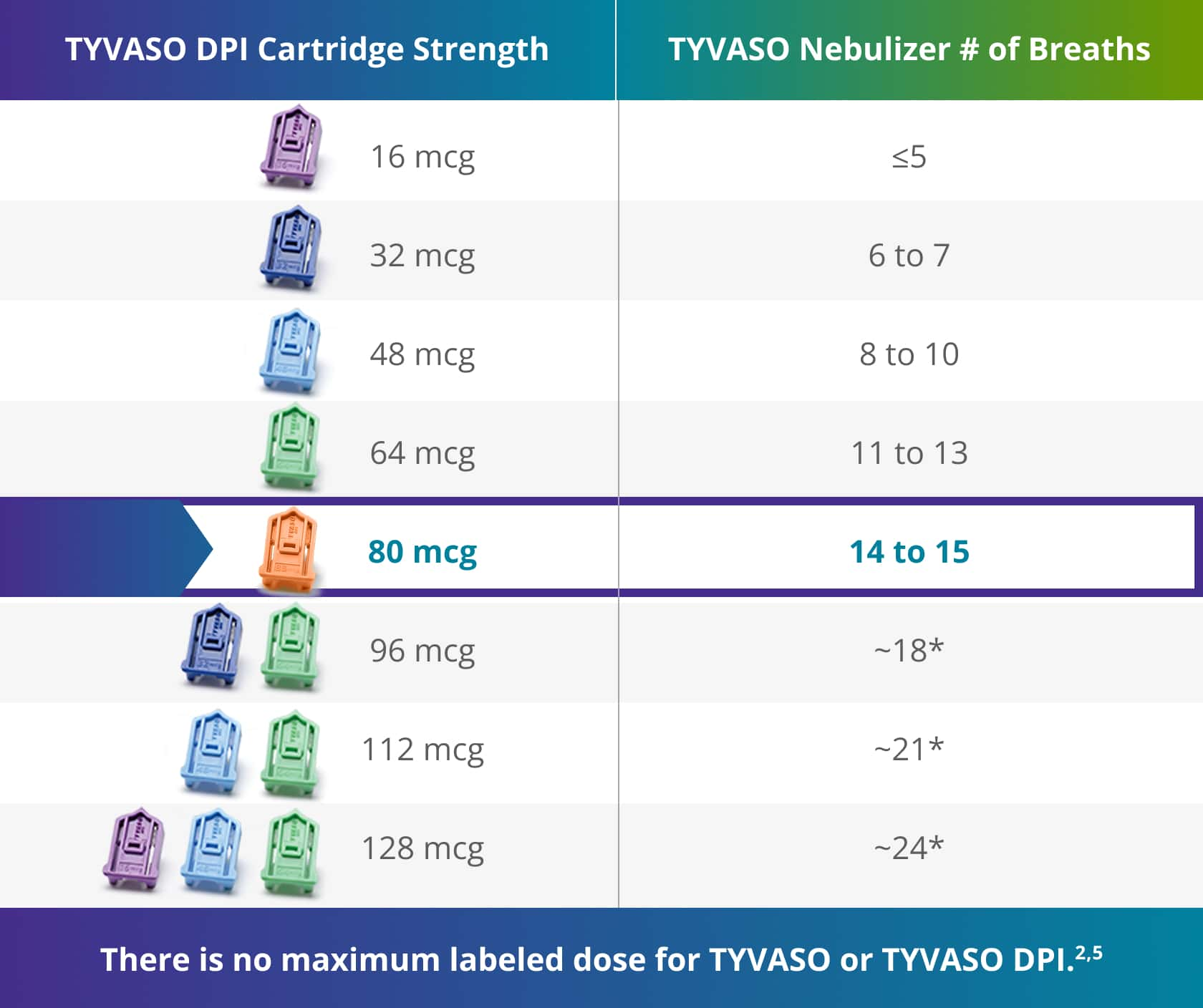
TYVASO DPI is the ONLY inhaler that can deliver a dose comparable to ~15 breaths of the TYVASO nebulizer with just 1 breath.2
*Based on extrapolation of lower doses assuming linearity.
Real-world dosing should be interpreted with appropriate caution. Time points are subject to patient attrition or discontinuation from treatment.
Real-world dosing in patients with PH-ILD starting de novo on TYVASO DPI
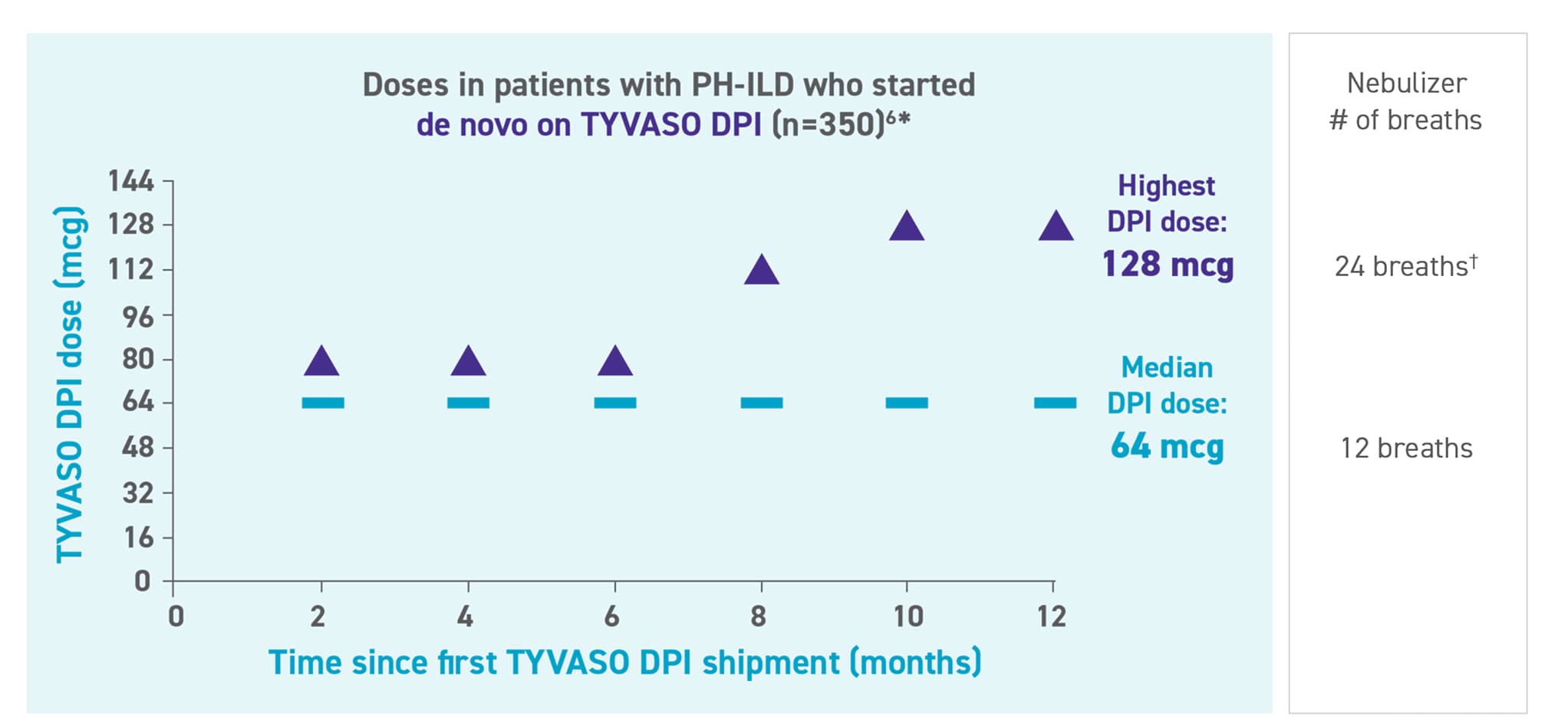
Specialty Pharmacy data were analyzed from patients starting de novo on TYVASO DPI.* Dosing was reported as shown in the shipment records and assessed in 2-month intervals to account for the variability of titration patterns.6
Patients with PH-ILD who transitioned to TYVASO DPI from the nebulizer (n=350) reached similar median and highest doses through month 12.6†
The majority of patients with PH-ILD using TYVASO DPI started de novo.1
*Specialty Pharmacy data were analyzed from patients with PH-ILD who received a first shipment of TYVASO DPI between May 25, 2022 and April 16, 2024. Patients (N=700) were randomly selected and stratified 1:1 by start type (ie, transition from the TYVASO nebulizer or de novo with no prior TYVASO shipment). Dosing was reported as shown in the shipment records and assessed in 2-month intervals to account for the variability of titration patterns. If multiple shipments were received within a 2-month interval, the highest dose shipment was used.6
†Comparison of 128 mcg TYVASO DPI to TYVASO nebulizer breaths is based on extrapolation of lower doses assuming linearity.
TYVASO nebulizer dosing & titration
Most patients achieved their optimal dose in 8 weeks7

75% of patients reached ≥9 breaths during the INCREASE study5
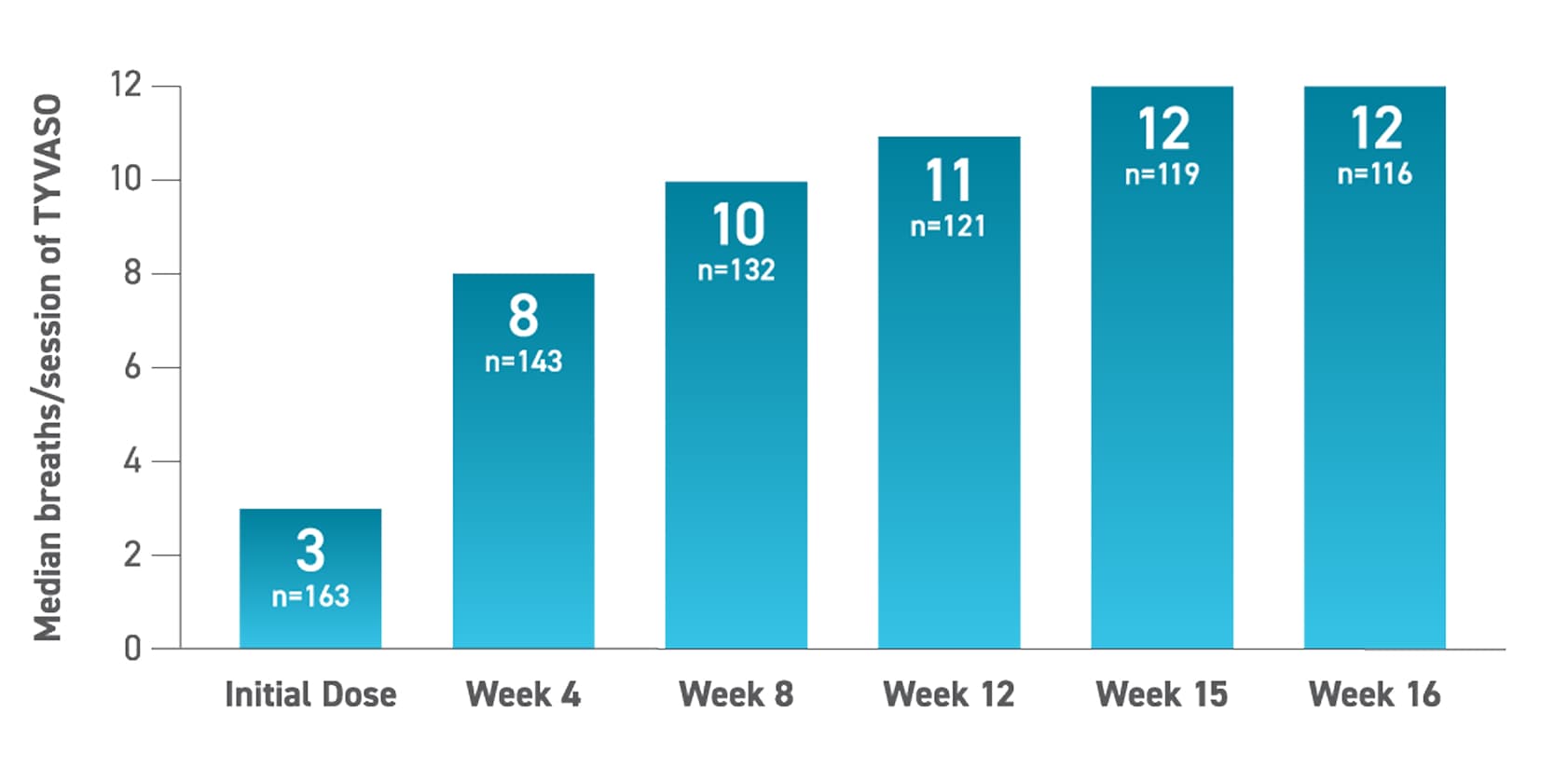
The INCREASE trial was designed to have a target dose of 9 breaths and a maximum dose of 12 breaths per treatment session.5
DPI=dry powder inhaler; PH-ILD=pulmonary hypertension associated with interstitial lung disease.
The information contained in this section of the site is clinical in nature and specifically created for healthcare professionals. If you are not a US healthcare professional, please click CLOSE to return to the consumer section of the site.