TYVASO is a prostacyclin mimetic indicated for the treatment of pulmonary arterial hypertension (PAH; WHO Group 1) to improve exercise ability. For the full TYVASO indication, see below.1
FREQUENTLY ASKED QUESTIONS
General TYVASO FAQs
What is Tyvaso?
HOW IS TYVASO DISTRIBUTED?
YOU ARE NOW LEAVING THE TYVASOHCP.COM WEBSITE
This link will take you to a site maintained by a third party who is solely responsible for the site’s content. Click CONTINUE to proceed or CLOSE to return to www.TYVASOhcp.com/pah.
YOU ARE NOW LEAVING THE TYVASOHCP.COM WEBSITE
This link will take you to a site maintained by a third party who is solely responsible for the site’s content. Click CONTINUE to proceed or CLOSE to return to www.TYVASOhcp.com/pah.
TYVASO is distributed by the following Specialty Pharmacies:
ACCREDO®
Phone: 1-866-344-4874
Fax: 1-800-711-3526
CVS SPECIALTY®
Phone: 1-877-242-2738
Fax: 1-877-943-1000
TYVASO can be prescribed by filling out the Referral Form.
CAN TYVASO BE USED DURING PREGNANCY?
There are no adequate and well-controlled studies with TYVASO in pregnant women. It is not known whether treprostinil is excreted in human milk.1
WHAT ADDITIONAL SUPPORT DOES THE SPECIALTY PHARMACY PROVIDER OFFER MY PATIENTS?
The Specialty Pharmacy providers are available to help initiate TYVASO therapy and provide continuous patient support with ongoing services and resources. Services offered to patients include one-step referral, reimbursement support, initial delivery of TYVASO and supplies, in-home device training, ongoing follow-up, and delivery of monthly refills.
What are the most common adverse events?
TRIUMPH I STUDY: Adverse events (AEs) observed in the 12-week, placebo-controlled study TRIUMPH I (N=235) at a rate of at least 4% and were more frequent in patients treated with TYVASO than placebo include1:
- Of the 23 total discontinuations, 7 patients discontinued in the TYVASO group due to adverse events compared with 4 in the placebo group2
- In addition, adverse events occurring in ≥10% of patients were dizziness and diarrhea1
TRIUMPH OLE STUDY: AEs observed during the chronic dosing study—in which 206 patients were dosed for a mean duration of 2.3 years, with a maximum exposure of 5.4 years—were qualitatively similar to those observed in the 12-week, placebo-controlled trial. Serious adverse events included pneumonia (n=15) and hemoptysis (n=3).1
BREEZE STUDY: During the 3-week treatment phase of the BREEZE study, most commonly reported (≥4% of patients) AEs in patients previously stable on TYVASO were3,4:
Discontinuation of TYVASO DPI due to an AE occurred in 2 (3.9%) patients.4
You may consider the following management approaches for common adverse events.
Selected AE management approaches5-7
The following approaches to managing adverse reactions are based on anecdotal evidence cited in Poms et al (2011)5 and should not be construed as medical advice. United Therapeutics does not recommend or endorse using healthcare products other than as directed or prescribed.
| Adverse Event | Management Approaches5-7 |
| Cough |
|
| Headache |
|
| Throat irritation |
|
Remind patients to talk to you or your staff about any adverse reactions and not to discontinue treatment without your direction.
TYVASO DPI FAQs
How can TYVASO DPI benefit my patients?
TYVASO DPI provides your patients with the benefits of TYVASO in a convenient delivery device, which is a simple-to-use option for the treatment of PAH.3,4
TYVASO DPI efficiently leverages a low flow rate to deliver medication evenly.8,9
A strong breath is not required to deliver a consistent dose to the distal airways.9
Is TYVASO DPI as safe and effective as TYVASO?
In a clinical study, patients experienced consistent safety and tolerability when switching to TYVASO DPI. Patient tolerability, as assessed by incidence of new adverse events following transition to TYVASO DPI, was consistent with the expected known safety profile of TYVASO. Please see the Full Prescribing Information for details.3
Systemic exposure was similar between TYVASO DPI and TYVASO.4,10
TYVASO DPI demonstrated a significant improvement of 11.5 m in 6MWD at week 3 compared with TYVASO treatment at baseline. Interim results of the optional extension phase suggest that this improvement was sustained over 51 weeks.4
What do patients think of TYVASO DPI?
Satisfaction with TYVASO DPI was higher when compared with the TYVASO nebulizer, with 98% (45/46) of patients reporting satisfaction at week 3 (P<0.0001) compared with 31% (16/51) of patients satisfied with the TYVASO nebulizer at baseline.4
How do I switch my patients from the TYVASO nebulizer to TYVASO DPI?
Patients currently using the TYVASO nebulizer can be switched to the corresponding dose of TYVASO DPI3:
Will my patients be able to inhale easily using the TYVASO DPI inhaler?
TYVASO DPI efficiently leverages a low flow rate to deliver medication evenly.8,9
A strong breath is not required to deliver a consistent dose to the distal airways.9
Do patients have to already be using TYVASO to transition to TYVASO DPI?
No, patients do not need to have an existing TYVASO prescription to start TYVASO DPI. Fill out a Referral Form to get your patients started with TYVASO DPI.
How do you use the TYVASO DPI inhaler?
TYVASO DPI is dosed with just 1 breath per cartridge, 4x daily. Each dose is inhaled in less than 2 seconds. Like the TYVASO nebulizer, TYVASO DPI treatment sessions can be scheduled around daily activities, approximately every 4 waking hours.3,8
How do the side effects of TYVASO DPI compare with TYVASO?
Patients experienced consistent safety and tolerability when switching to TYVASO DPI. Patient tolerability, as assessed by incidence of new adverse events following transition to TYVASO DPI, was consistent with the expected known safety profile of TYVASO. Please see the Full Prescribing Information for details.3
During the 3-week treatment phase of the BREEZE study, most commonly reported (≥4% of patients) AEs in patients previously stable on TYVASO were3,4:
How is TYVASO DPI stored?
Blister cards and strips:
Store at room temperature, 59°F to 86°F (15°C to 30°C). When at room temperature, sealed and unopened blister cards and strips must be used within 8 weeks, and opened strips must be used within 3 days.3
Additional blister cards and strips may be stored in the refrigerator, 36ºF to 46ºF (2ºC to 8ºC), until the expiration date printed on the blisters, but should be room temperature for 10 minutes before use.3
Do not put a blister card or strip back into the refrigerator after being opened or stored at room temperature.3
Inhaler:
The TYVASO DPI inhaler may be stored at room temperature or in the refrigerator, 36ºF to 77ºF (2ºC to 25ºC). If refrigerated, the inhaler should be left at room temperature for 10 minutes before use.3
The inhaler can be used for up to 7 days from the date of first use. After 7 days of use, the inhaler must be discarded and replaced with a new inhaler.3
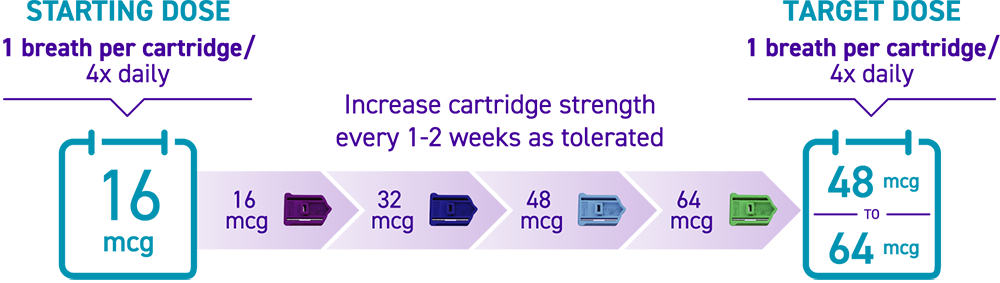
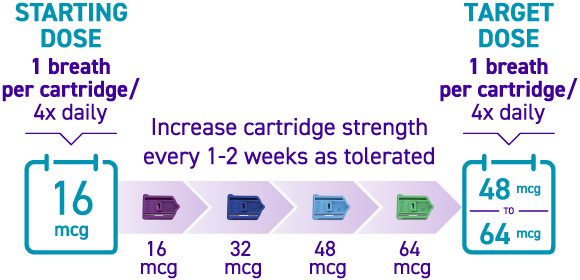
How is TYVASO DPI titrated?
Titration with TYVASO DPI3:
Titration schedules may vary based on tolerability. If the prescribed dose is higher than 64 mcg per treatment session, more than 1 cartridge will be needed per session.3
How is TYVASO DPI covered under Medicare?
While the TYVASO nebulizer is covered under Medicare Part B, TYVASO DPI is covered under Medicare Part D. Part D premiums vary by plan.
TYVASO NEBULIZER FAQS
How was Tyvaso studied?
TYVASO was studied in TRIUMPH, a combination trial in PAH. TRIUMPH I was a 12-week, multicenter, randomized, double-blind, placebo-controlled trial of an inhaled prostacyclin (TYVASO) and a single oral PAH agent (bosentan or sildenafil). The primary efficacy endpoint of the trial was the change in 6-minute walk distance (6MWD) relative to baseline at 12 weeks.1,2
TYVASO was also studied in an open-label extension study, TRIUMPH OLE, to evaluate the efficacy, safety, and dosing of TYVASO over time. Patients (N=206) from the placebo-controlled study initiated TYVASO after completing the 12-week TRIUMPH study, entering the long-term, uncontrolled OLE study.1,11
Are there any data on long-term treatment with Tyvaso?
The long-term use and safety of TYVASO have been studied in an open-label extension of the pivotal TRIUMPH I study, with a mean duration of 2.3 years and maximum exposure of 5.4 years (N=206).1
Safety and dosing were evaluated:
- 89% of patients achieved the target dose of 9 breaths, 4x daily1
- 42% of patients achieved a dose of 12 breaths, 4x daily1
- The maximum recommended dosage is 9 breaths, 4x daily1
The adverse events during this chronic dosing study were qualitatively similar to those observed in the 12-week, placebo-controlled trial. Additionally, serious adverse events included pneumonia (n=15) and hemoptysis (n=3).1
Kaplan–Meier estimates of survival were1:
- 97% at 1 year
- 91% at 2 years
- 82% at 3 years
These uncontrolled observations do not allow comparison with a control group not given TYVASO and cannot be used to determine the long-term effect of TYVASO on mortality.1
What is the Tyvaso nebulizer?
The TYVASO nebulizer is a lightweight, portable, handheld device approved for direct-to-the-lungs administration of TYVASO.1
How long does each dosing session with the TYVASO Nebulizer take?
Each dosing session with the TYVASO nebulizer should take about 2-3 minutes and should be administered 4x daily, during waking hours.1
How do patients keep track of their treatments?
Patients using the TYVASO nebulizer can use the TYVASO Treatment Tracker to help keep track of breaths during each treatment session. A new treatment tracker is provided monthly with each TYVASO prescription refill.
How long does it take to set up the Tyvaso nebulizer?
Setting up the TYVASO nebulizer for use takes about 5 minutes and must be done once each day prior to the day’s first treatment session.12
How is the Tyvaso nebulizer cleaned?
The TYVASO nebulizer requires once-daily cleaning with warm, soapy water. Accessory parts should be washed by hand and allowed to air-dry. The base of the device should not be immersed in water or placed in a dishwasher.6
Once a week, use a clean cloth to wipe the interior of the device chamber. For more instructions on cleaning and maintenance of the TYVASO nebulizer, refer to the Cleaning and Storing section beginning on page 46 of the TYVASO Nebulizer Instructions for Use manual.6
How do you order and replace the accessories?
For patient convenience, replacement accessory parts are delivered monthly with each TYVASO prescription refill. Call your Specialty Pharmacy provider with any additional questions. Patients will receive a replacement device every 2 years.
How is Tyvaso supplied?
TYVASO is supplied in 2.9-mL, clear, low-density polyethylene (plastic) ampules packaged as 4 ampules in a foil pouch. TYVASO is a clear, colorless to slightly yellow solution containing 1.74 mg of treprostinil per ampule at a concentration of 0.6 mg/mL.1
The TYVASO Nebulizer Starter Kit contains a 28-ampule carton of TYVASO (the 7 foil pouches each contain four 2.9-mL ampules; each ampule contains 1.74 mg treprostinil [0.6 mg per mL]) and 2 TYVASO nebulizers (NDC 66302-206-01).1
The TYVASO Nebulizer Refill Kit contains a 28-ampule carton of TYVASO (the 7 foil pouches each contain four 2.9-mL ampules; each ampule contains 1.74 mg treprostinil [0.6 mg per mL]) and accessories (NDC 66302-206-02).1
The TYVASO 4-Pack Carton with 1 foil pouch contains four 2.9-mL ampules. Each ampule contains 1.74 mg treprostinil (0.6 mg per mL) (NDC 66302-206-03).1
The TYVASO Nebulizer Institutional Starter Kit contains a 4-ampule carton of TYVASO (1 foil pouch contains four 2.9-mL ampules; each ampule contains 1.74 mg treprostinil [0.6 mg per mL]) and 2 TYVASO nebulizers (NDC 66302-206-04).1
How is Tyvaso stored?
Ampules of TYVASO are stable until the date indicated when stored in the unopened foil pouch at 25ºC (77ºF), with excursions permitted to 15ºC to 30ºC (59ºF to 86ºF) [see USP Controlled Room Temperature]. Once the foil pack is opened, ampules should be used within 7 days. Because TYVASO is light-sensitive, unopened ampules should be stored in the foil pouch.1
One ampule of TYVASO should be used each day in the TYVASO nebulizer. After a TYVASO ampule is opened and transferred to the medicine cup, the solution should remain in the device for no more than 1 day (24 hours). Any remaining solution should be discarded at the end of the day.1